Gururaj Joshi, Anthony P. Davis*
Org. Biomol. Chem., 2012, 10, 5760-5763
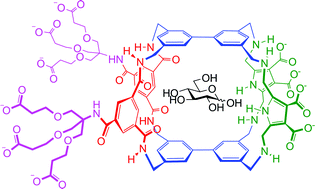
Abstract
Synthetic lectins are molecules designed for the challenging task of biomimetic carbohydrate recognition in water. Previous work has explored a family of such systems based on bi/terphenyl units as hydrophobic surfaces and isophthalamide spacers to provide polar binding groups. Here we report a related receptor which employs a new spacer, 2,5-bis-(aminomethyl)-pyrrole, with an alternative (A-D-A) set of H-bonding valencies. The modified spacer leads to significant changes in binding selectivity, including a preference for glucose over all other tested substrates.