E. Klein, Y. Ferrand, N. P. Barwell, A. P. Davis*
Angew. Chem. Int. Ed. 2008, 48, 2693-2696
Abstract
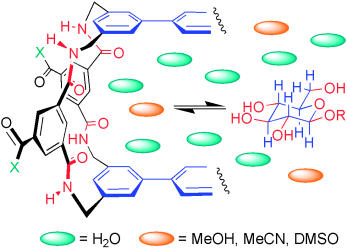
How do they do it? Carbohydrates are intrinsically difficult to bind in water. Lectins and other proteins succeed, but do they use polar or hydrophobic interactions? Studies of synthetic lectins in mixed solvent systems shed new light onto this contentious issue (see scheme; X=hydrophobic or hydrophilic solubilizing groups; R=H or alkyl group).