V. del Amo, A. P. McGlone, J.-M. Soriano, A. P. Davis*
Tetrahedron 2009, 65, 6370-6381
Abstract
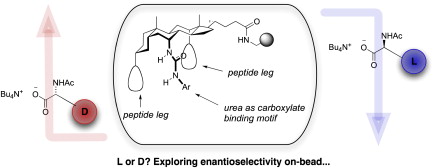
The screening of resin-bound combinatorial libraries with pairs of dye-tagged substrates is a powerful strategy for discovering selective receptors. However, implementation has been hampered by a lack of complementary but chemically similar dyes. We now show that the well-established Disperse Red 1 and the recently-introduced Bristol Blue 1 can be used in parallel to synthesise ‘pseudoenantiomeric’ analogues of N-acetyl-α-amino acids and of the anti-inflammatory drug Naproxen. A steroid-based receptor library has been prepared and screened with these substrates. Preliminary results suggest that some members may be highly enantioselective receptors for N-acetyl-α-amino acids.