Robert A. Tromans, Tom S. Carter, Laurent Chabanne, Matthew P. Crump, Hongyu Li, Johnathan V. Matlock, Michael G. Orchard and Anthony P. Davis
Abstract
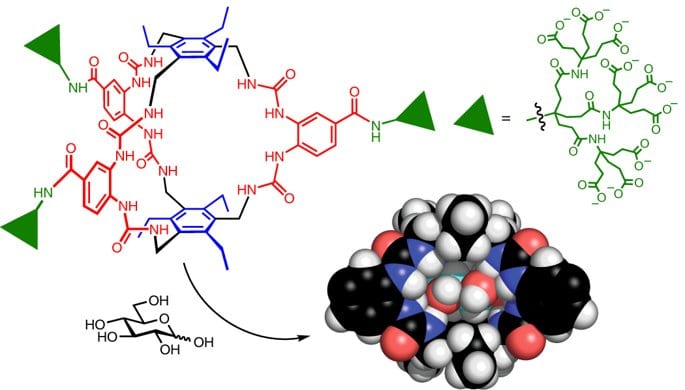
Specific molecular recognition is routine for biology, but has proved difficult to achieve in synthetic systems. Carbohydrate substrates are especially challenging, because of their diversity and similarity to water, the biological solvent. Here we report a synthetic receptor for glucose, which is biomimetic in both design and capabilities. The core structure is simple and symmetrical, yet provides a cavity which almost perfectly complements the all-equatorial β-pyranoside substrate. The receptor’s affinity for glucose, at Ka ~ 18,000 M−1, compares well with natural receptor systems. Selectivities also reach biological levels. Most other saccharides are bound approximately 100 times more weakly, while non-carbohydrate substrates are ignored. Glucose-binding molecules are required for initiatives in diabetes treatment, such as continuous glucose monitoring and glucose-responsive insulin. The performance and tunability of this system augur well for such applications.