Ramalingam Natarajan, Lydia Bridgland, Anchalee Sirikulkajorn, Ji-Hun Lee, Mairi F. Haddow, Germinal Magro, Bakhat Ali, Sampriya Narayanan, Peter Strickland, Jonathan P. H. Charmant, A. Guy Orpen, Neil B. McKeown, C. Grazia Bezzu, and Anthony P. Davis*
J. Am. Chem. Soc. 2013, 135, 16912–16925
Abstract
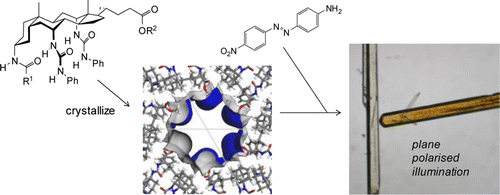
Previous work has shown that certain steroidal bis-(N-phenyl)ureas, derived from cholic acid, form crystals in the P61 space group with unusually wide unidimensional pores. A key feature of the nanoporous steroidal urea (NPSU) structure is that groups at either end of the steroid are directed into the channels and may in principle be altered without disturbing the crystal packing. Herein we report an expanded study of this system, which increases the structural variety of NPSUs and also examines their inclusion properties. Nineteen new NPSU crystal structures are described, to add to the six which were previously reported. The materials show wide variations in channel size, shape, and chemical nature. Minimum pore diameters vary from 0 up to 13.1 Å, while some of the interior surfaces are markedly corrugated. Several variants possess functional groups positioned in the channels with potential to interact with guest molecules. Inclusion studies were performed using a relatively accessible tris-(N-phenyl)urea. Solvent removal was possible without crystal degradation, and gas adsorption could be demonstrated. Organic molecules ranging from simple aromatics (e.g., aniline and chlorobenzene) to the much larger squalene (Mw = 411) could be adsorbed from the liquid state, while several dyes were taken up from solutions in ether. Some dyes gave dichroic complexes, implying alignment of the chromophores in the NPSU channels. Notably, these complexes were formed by direct adsorption rather than cocrystallization, emphasizing the unusually robust nature of these organic molecular hosts.