A. Sirikulkajorn, T. Tuntulani, V. Ruangpornvisuti, B. Tomapatanaget, A. P. Davis*
Tetrahedron, 2010, 66, 7423-7428
Abstract
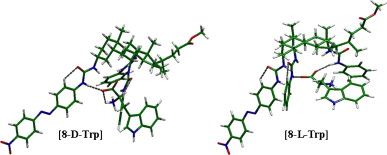
A cholapod receptor possessing urea binding sites at C3, C7, and C12 positions and with an intrinsic chiral structure was synthesized, and the binding abilities toward amino acids in both l- and d- forms (Trp, Phe, Leu, and Ala) were studied using 1H NMR spectroscopy, UV–vis spectroscopy and computer simulation. Changes in 1H NMR spectra of the receptor revealed that complexation with amino acids occurred via hydrogen bonding and CH–π interactions. Binding to tryptophan was especially strong, and was found to be enantioselective (Ka=480 M−1 for l-Trp, 260 M−1 for d-Trp). NOESY and computer simulations were used to investigate the structures of the diastereomeric complexes between the receptor and the tryptophan enantiomers. In the case of l-Trp the carboxylate group bound at the two ureas adjacent to C7 and C12, while d-Trp was positioned closer to the urea adjacent to C3..