Sophie J. Edwards, Igor Marques, Christopher M. Dias, Robert A. Tromans, Nicholas R. Lees, Vítor Félix*, Hennie Valkenier* and Anthony P. Davis*
Chem. Eur. J. 2016, 22, 2004-2011
Abstract
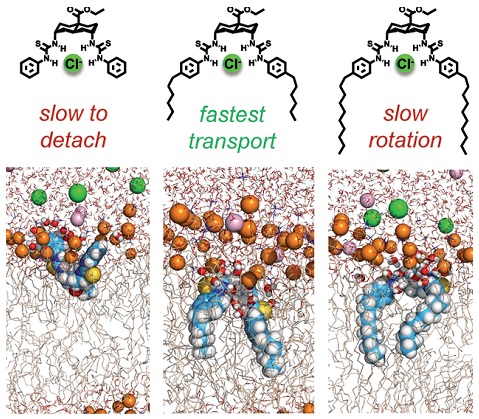
Anion transport by synthetic carriers (anionophores) holds promise for medical applications, especially the treatment of cystic fibrosis. Among the factors which determine carrier activity, the size and disposition of alkyl groups is proving remarkably important. Herein we describe a series of dithioureidodecalin anionophores, in which alkyl substituents on one face are varied from C0 to C10 in two-carbon steps. Activities increase then decrease as the chain length grows, peaking quite sharply at C6. Molecular dynamics simulations showed the transporter chloride complexes releasing chloride as they approach the membrane-aqueous interface. The free transporter then stays at the interface, adopting an orientation that depends on the alkyl substituent. If chloride release is prevented, the complex is positioned similarly. Longer chains tilt the binding site away from the interface, potentially freeing the transporter or complex to move through the membrane. However, chains which are too long can also slow transport by inhibiting movement, and especially reorientation, within the phospholipid bilayer.