Y. Ferrand, E. Klein, N. P. Barwell, M. P. Crump, J. Jiménez-Barbero, C. Vicent, G.-J. Boons, S. Ingale, A. P. Davis*
Angew. Chem. Int. Ed. 2009, 49, 1775
This paper was selected to feature on an inside cover
Abstract
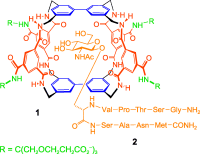
Changing employment: Receptor 1 binds β-N-acetylglucosaminyl (β-GlcNAc) up to 100 times more strongly than it does glucose. This synthetic lectin shows affinities similar to wheat germ agglutinin (WGA), a natural lectin used to bind GlcNAc. Remarkably, 1 is more selective than WGA. It favors especially the glycoside unit in glycopeptide 2, a model of the serine-O-GlcNAc posttranslational protein modification.