Tom S. Carter, Tiddo J. Mooibroek, Patrick F. N. Stewart, Matthew P. Crump, M. Carmen Galan and Anthony P. Davis*
Angew. Chem. Int. Ed. 2016, 55, 9311-9315
Abstract
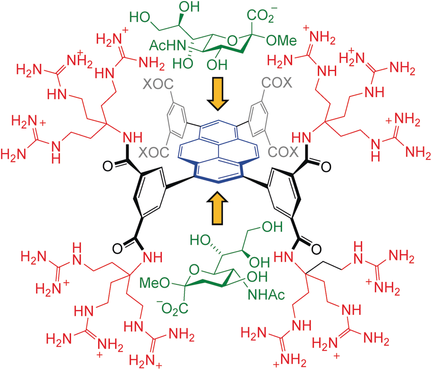
Biomimetic carbohydrate receptors (“synthetic lectins”) have potential as agents for biological research and medicine. However, although effective strategies are available for “all-equatorial” carbohydrates (glucose, etc.), the recognition of other types of saccharide under natural (aqueous) conditions is less well developed. Herein we report a new approach based on a pyrene platform with polar arches extending from aryl substituents. The receptors are compatible with axially substituted carbohydrates, and also feature two identical binding sites, thus mimicking the multivalency observed for natural lectins. A variant with negative charges forms 1:2 host/guest complexes with aminosugars, with K1 > 3000 M−1 for axially substituted mannosamine, whereas a positively charged version binds the important α-sialyl unit with K1 ≈ 1300 M−1.