Federica Balduzzi, Patrick Stewart, Dr. Soumen K. Samanta, Dr. Tiddo J. Mooibroek, Dr. Thomas Hoeg-Jensen, Kejia Shi, Prof. Dr. Bradley D. Smith, Prof. Dr. Anthony P. Davis
Angew. Chem. Int. Ed. 2023, e202314373
Abstract
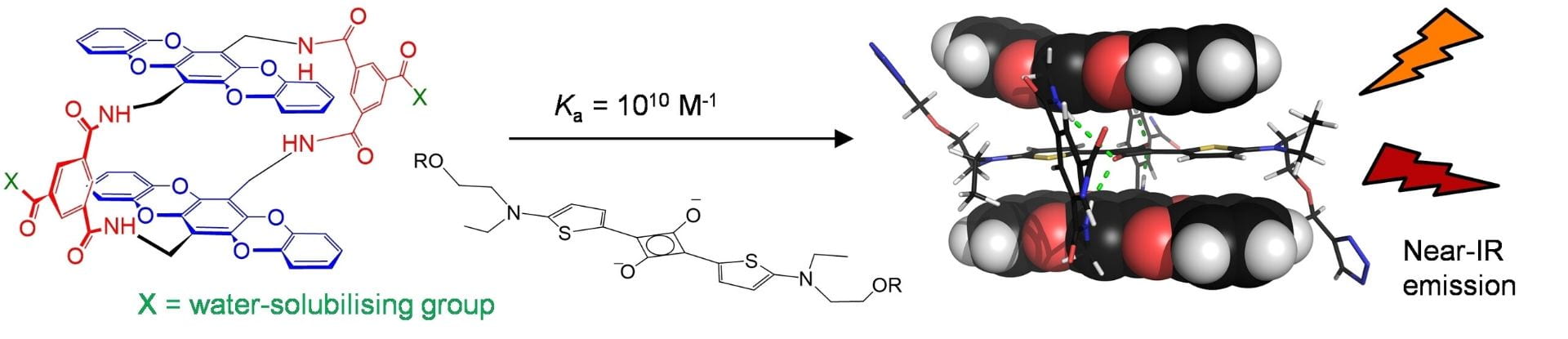
Strong-binding host–guest pairings in aqueous media have potential as “supramolecular glues” in biomedical techniques, complementing the widely-used (strept)avidin-biotin combination. We have previously found that squaraine dyes are bound very strongly by tetralactam macrocycles possessing anthracenyl units as cavity walls. Here we show that replacing the anthracenes with pentacyclic 5,7,12,14-tetrahydro-5,7,12,14-tetraoxapentacene (TOP) units generates receptors which bind squaraines with increased affinities (around Ka=1010 m−1) and improved selectivities. Binding can be followed through changes to squaraine fluorescence and absorbance. The TOP units are easy to prepare and potentially variable, while the TOP-based receptor shows improved photostability, both in itself and in complex with squaraines. The results suggest that this system could prove valuable in the further development of practical “synthavidin” chemistry.