Pablo Ríos, Tiddo J. Mooibroek,* Tom S. Carter, Christopher Williams, Miriam R. Wilson, Matthew P. Crump and Anthony P. Davis*
Chem. Sci. 2017, 8, 4056-4061.
Abstract
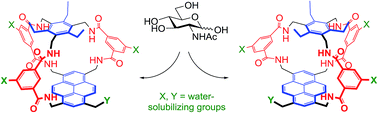
Carbohydrate receptors with a chiral framework have been generated by combining a tetra-aminopyrene and a C3-symmetrical triamine via isophthalamide spacers bearing water-solubilising groups. These “synthetic lectins” are the first to show enantiodiscrimination in aqueous solution, binding N-acetylglucosamine (GlcNAc) with 16 : 1 enantioselectivity. They also show exceptional affinities. GlcNAc is bound with Ka up to 1280 M−1, more than twice that measured for previous synthetic lectins, and three times the value for wheat germ agglutinin, the lectin traditionally employed to bind GlcNAc in glycobiological research. Glucose is bound with Ka = 250 M−1, again higher than previous synthetic lectins. The results suggest that chirality can improve complementarity to carbohydrate substrates and may thus be advantageous in synthetic lectin design.