Sophie J. Edwards, Hennie Valkenier, Nathalie Busschaert, Philip A. Gale* and Anthony P. Davis*
Angew. Chem. Int. Ed., 2015, 54, 4592-4596
Abstract
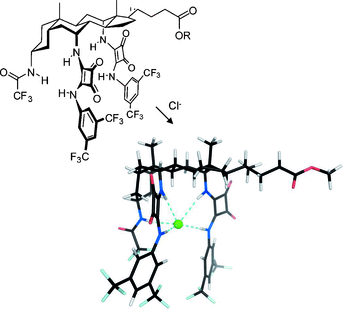
Exceptionally powerful anion receptors have been constructed by placing squaramide groups in axial positions on a steroidal framework. The steroid preorganizes the squaramide NH groups such that they can act cooperatively on a bound anion, while maintaining solubility in nonpolar media. The acidic NH groups confer higher affinities than previously-used ureas or thioureas. Binding constants exceeding 1014 m−1 have been measured for tetraethylammonium salts in chloroform by employing a variation of Cram’s extraction procedure. The receptors have also been studied as transmembrane anion carriers in unilamellar vesicles. Unusually their activities do not correlate with anion affinities, thus suggesting an upper limit for binding strength in the design of anion carriers.