Chenfeng Ke, Ronald A. Smaldone, Takashi Kikuchi, Hao Li, Prof. Anthony P. Davis, Prof. J. Fraser Stoddart*
Angew. Chem. Int. Ed. Engl. 2013, 52, 381-387
Abstract
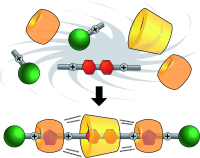
In one fell swoop, polyrotaxanes comprising up to 64 rings can be synthesized as a result of cucurbit[6]uril-templated 1,3-dipolar azide-alkyne cycloadditions accelerated in the presence of cyclodextrins as a consequence of self-sorting and positive cooperativity, brought about by hydrogen bonding. Mixing six components (see picture) in one pot affords a hetero[4]rotaxane in one minute in quantitative yield.