F. Corzana, A. Fernandez-Tejada, J. H. Busto, G. Joshi, A. P. Davis, J. Jimenez-Barbero, A. Avenoza, J. M. Peregrina*
ChemBioChem, 2011, 12, 110-117
Abstract
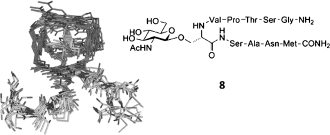
The binding properties of different carbohydrates and glycopeptides containing the β-O-2-deoxy-2-(N-acetyl)-d-glucosaminyl (β-O-GlcNAc) to a synthetically prepared lectin-like receptor have been analyzed. The study combines the use of NMR spectroscopy experiments with extensive MD simulations in explicit water. Notably, the presence of a key hydrogen bond between the receptor and the OMe group of the β-O-GlcNAc-OMe derivative appears to be responsible for the high selectivity observed for this compound. In addition, to study the effect on the binding of the underlying amino acid, we have prepared different model glycopeptides, which include the non-natural α-methylserine and α-methylthreonine as underlying amino acids. Interestingly, the presence of a methyl group decreases the affinity constant, especially in those cases in which a β-methyl group is present. As a result, the serine-containing glycopeptide exhibited the highest affinity constant of the glycopeptides, and the threonine derivative showed the lowest one. This low selectivity could have its origin in the difficulty to form both specific hydrogen bonds and hydrophobic (CH-π) contacts.