K. M. Bhattarai, V. del Amo, G. Magro, A. L. Sisson, J.-B. Joos, J. P. H. Charmant, A. Kantacha, and A. P. Davis*
Abstract
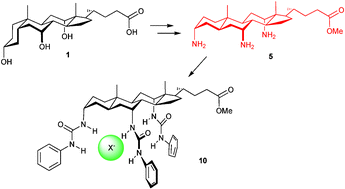
Cholic acid 1 has been converted into triamine 5 with the all-trans polycyclic allocholanoyl skeleton and co-directed, axial amino groups; the potential of this system as a scaffold is illustrated by conversion to a preorganised anion receptor.