Ondřej Jurček, Hennie Valkenier, Rakesh Puttreddy, Martin Novák, Hazel A. Sparkes, Radek Marek, Kari Rissanen and Anthony P. Davis
Chem. Eur. J. 2018, 24, 8178-8185.
Abstract
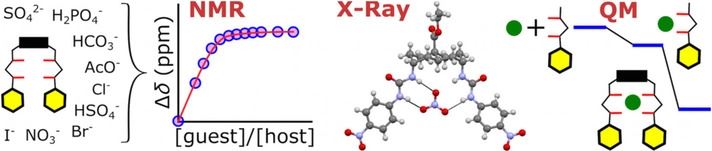
Recent work has identified a bis‐(p‐nitrophenyl)ureidodecalin anion carrier as a promising candidate for biomedical applications, showing good activity for chloride transport in cells yet almost no cytotoxicity. To underpin further development of this and related compounds, a detailed structural and binding investigation is reported. Crystal structures of the transporter as five solvates confirm the diaxial positioning of urea groups while revealing a degree of conformational flexibility. Structures of complexes with Cl−, Br−, NO3−, SO42− and AcO−, supported by computational studies, show how the binding site can adapt to accommodate these anions. 1H NMR binding studies revealed exceptionally high affinities for anions in DMSO, decreasing in the order SO42−>H2PO4−≈HCO3−≈AcO−≫HSO4−>Cl−>Br−>NO3−>I−. Analysis of the binding results suggests that selectivity is determined mainly by the H‐bond acceptor strength of different anions, but is also modulated by receptor geometry.