Pablo Rios, Tom S. Carter, Tiddo J. Mooibroek*, Matthew P. Crump, Micke Lisbjerg, Michael Pittelkow, Nitin T. Supekar, Geert-Jan Boons and Anthony P. Davis*
Angew. Chem. Int. Ed., 2016, 55, 3387-3392
Abstract
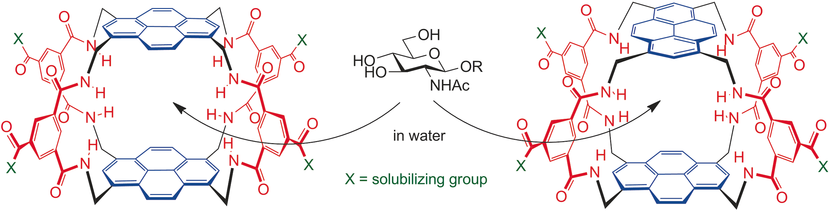
The combination of a pyrenyl tetraamine with an isophthaloyl spacer has led to two new water-soluble carbohydrate receptors (“synthetic lectins”). Both systems show outstanding affinities for derivatives of N-acetylglucosamine (GlcNAc) in aqueous solution. One receptor binds the methyl glycoside GlcNAc-β-OMe with Ka≈20,000 M−1, whereas the other one binds an O-GlcNAcylated peptide with Ka≈70,000 M−1. These values substantially exceed those usually measured for GlcNAc-binding lectins. Slow exchange on the NMR timescale enabled structural determinations for several complexes. As expected, the carbohydrate units are sandwiched between the pyrenes, with the alkoxy and NHAc groups emerging at the sides. The high affinity of the GlcNAcyl–peptide complex can be explained by extra-cavity interactions, raising the possibility of a family of complementary receptors for O-GlcNAc in different contexts.