N. P. Barwell, A.P. Davis*
J. Org. Chem., 2011, 76, 6548–6557
Abstract
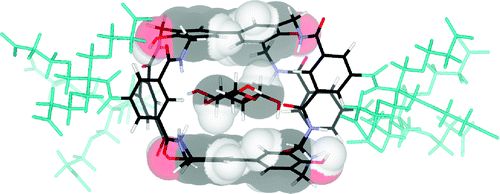
Contacts between aromatic surfaces and saccharide CH groups are common motifs in natural carbohydrate recognition. These CH−π interactions are modeled in “synthetic lectins” which employ oligophenyl units as apolar surfaces. Here we report the synthesis and study of new synthetic lectins with fluoro- and hydroxy-substituted biphenyl units, designed to explore the role of π-electron density in carbohydrate CH−π interactions. We find evidence that recognition can be moderated through electronic effects but that other factors such as cavity hydration are also important and sometimes predominant in determining binding strengths.