M. R. M. Andreae, A. P. Davis*
Tetrahedron-Asymmetry, 2005, 16, 2487–2492
Abstract
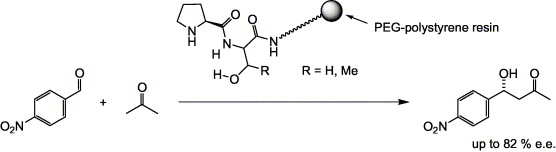
Peptides with prolyl N-termini, attached to a PEG–polystyrene (TG) synthesis resin, have been tested as heterogeneous catalysts for the aldol reaction between acetone and p-nitrobenzaldehyde. Proline directly attached to TG showed good activity but poor enantioselectivity. However, in combination with serine or threonine, the selectivity improved considerably. At −25 °C, the dipeptide H-Pro-Ser-NH-TG achieved 82% ee. The H-Pro-Ser/Thr dipeptides may be seen as self-contained ‘catalytic head-groups’ for the development of more sophisticated aldol organocatalysts.